New research suggests that fibroblasts may play two roles in repairing skin injury—not only rebuilding the skin but also removing damaged tissue.
The findings were published in APL Bioengineering.
Researchers at Boston University and Harvard University uncovered this cellular capability by simulating both cuts and burns in a miniature skin tissue model and monitoring how fibroblasts responded. The investigators found that in scorched samples, fibroblasts consumed and removed damaged tissue. They write that until now this process was thought to be solely carried out by immune cells. The authors of the study suggest that the finding is an important first step toward the development of future therapies that speed up and optimize the healing of burns.
“Our bodies need to clear dead tissue out of wounds before it begins repair. But if that phase takes too long, then the wound won’t repair at all,” said the study’s lead author Jeroen Eyckmans, PhD, in a press release. “You want to shorten that first period as much as possible.”
Dr. Eyckmans is a professor at Boston University’s College of Engineering.
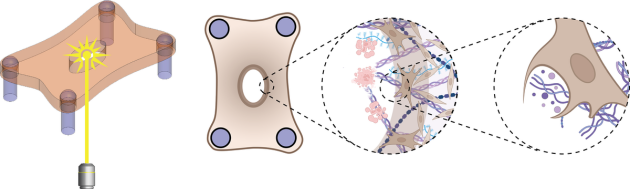
To investigate the wound healing process, Dr. Eyckmans and his colleagues previously engineered a microtissue model composed of fibroblasts buried within a tiny sheet composed of collagen.
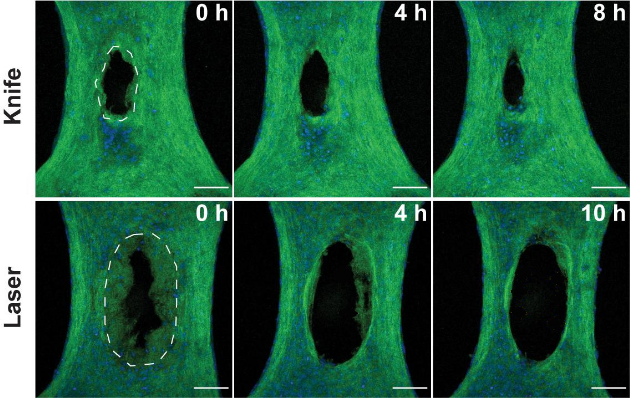
Initially, the researchers created wounds in the model exclusively with a one-half-millimetre-wide diamond blade, however, the lacerations were so small, it was difficult to examine how the cells responded. In the new study, they used lasers to simulate burn injuries that would be larger and more straightforward to study.
The researchers compared how the cells responded in both injury types by assessing microscopic images collected in the hours following the injuries. They noticed many similarities between the two wound types but several key differences as well.
While the knife damaged the tissue immediately at the site of the incision—sparing the cells and extracellular matrix (ECM) adjacent to the wound—heat generated by the laser created a ring of damaged tissue around where the laser struck.
The knife wound began closing within minutes as expected, but the burn began widening over many hours. The researchers write they were caught by surprise by the removal of damaged tissue in the burns, as their model excluded any immune cells, which normally lead the charge in removing damaged tissue.
Close inspection revealed that fibroblasts were clearing out the ECM damaged by the laser. Further experiments involving the inhibition of specific proteins within the fibroblasts suggested that the cells were ingesting ECM.
“To my knowledge, this is the first study showing that fibroblasts can exhibit phagocytosis in a wound healing context,” Dr. Eyckmans said. “Now we have an additional cell type to target that could speed up wound clearance.”
Investigators write that their findings suggest future clinical treatments could potentially coax fibroblasts to initiate or accelerate ECM removal, facilitating burn healing during the critical early phase.
The laser injury model permitted Dr. Eyckmans and his colleagues to take a first look at wound clearance at the cellular level, but the team does not intend to stop there. They plan to extend their model to examine how other cell types, such as those in the vasculature, clear and mend wounds.


Comments